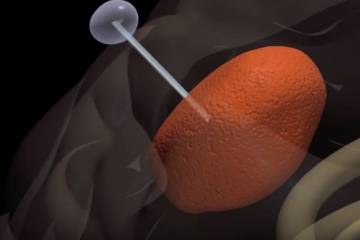
The U.S. Food and Drug Administration (FDA) recently bestowed a “Breakthrough Device” designation for a tube-like device that is designed to attract brain tumor cells such as glioblastoma. One of the major challenges associated with either surgical or radiotherapy treatment for glioblastoma is that tumor cells migrate and invade the brain far away from the main tumor mass. Hence
The device, known as the Tumor Monorail, should permit management of such tumors and prevent such tumors from taking over the brain. With the FDA’s “Breakthrough Device” label, this item will receive a priority to review drug-manufacturing candidates to commence clinical trials.
Since the device attracts tumor cells, researchers are exploring ways to incorporate drugs that can kill cancer cells into the device.
From the Duke’s Research Leader, who previously had launched studying the Tumor Monorail while at the Georgia Tech. [Read details on a Duke post, “Brain Tumor ‘Pied Piper’ Device Gains Breakthrough Status”]:
“What’s most important is that the tumor is spreading in a controlled way through our device to a reservoir, and away from the mother tumor, rather than through the healthy brain tissue. The toxic gel inside the reservoir appears to only play a secondary role, though additional preclinical studies will help make this clear. Simply by being far away from the mother tumor, the cells are more susceptible to dying anyway, and a neurosurgeon can access the reservoir to empty it when needed.”
Nassir Mokarram, a research faculty member in the Department of Biomedical Engineering at Duke University and leader of the project mentioned above
While the current device has only been tested on mice, the researchers are hoping that the FDA will approve human trials by the end of this year, 2019. The most exciting finding with this device was that the spread of the tumor diminished and even shrank by 90%. We will share further information as research continues.